Table of Contents
Introduction: Dracula, Immortality, and Our Obsession With Staying Young
I was recently in New Orleans in March 2025 where I was invited to speak in conference. Given the intriguing history of New Orleans, this conference gave me the perfect opportunity to tour the city and immerse myself in dark stories.
The “Ghost and Vampire Walking Tour of the French Quarter” was surprisingly very good—reasonably priced, not overcrowded, yet filled with jaw-dropping stories and legends. Lot of stories intrigued me. But as we shuffled past wrought-iron balconies and gas-lit alleyways, our guide dropped a story that made my “medical” brain start ticking.
Jacques St. Germain. Ever heard of him? Probably not. He was this enigmatic French aristocrat who arrived in New Orleans sometime in the early 1900s. He was wealthy, charming, and most notably- unnaturally youthful for his apparent age. The locals whispered that he never aged, that he sustained himself by drinking the blood of young women.
It sounds suspiciously familiar, doesn’t it? The myth of Count Dracula—hunting in the darkness of the night, feeding on human blood to stave off the rot of time. Both men were creepy predators, who would come out in the dark and hunt for human blood. One could sense an uncomfortable symmetry here- mythologies from two different cultures, from two different generations, from two different continents. Yet both share the same recipe to remain young- feeding on young blood!
I should have laughed it off. But something about that particular legend stuck with me as I walked back to my hotel that night. Maybe it was the timing. I had just been reading papers on parabiosis research. These are scientific studies where researchers literally stitch young and old mice together to share blood circulation. The results are wild: the old mice start showing signs of cellular rejuvenation, improved organ function, even cognitive enhancement.
Then it hit me. What if these vampire stories weren’t just medieval superstition? What if they were primitive observations of real biological phenomena?
Think about it. For centuries, folklore across cultures has obsessed over this same theme: the old feeding on the young to maintain vitality. From Elizabeth Báthory bathing in virgin blood to modern vampire fiction, the pattern is consistent. Too consistent to be coincidence.
And now, in 2025, we’re discovering that young blood contains factors that can literally reverse aging in mammals. Companies are popping up offering “young plasma” transfusions. Researchers are isolating proteins in young blood that restore muscle mass, improve memory, and repair damaged tissue in older organisms.
The vampire’s ancient hunger might not have been supernatural at all. It might have been biology that we’re only now sophisticated enough to understand.
What started as tourist schlock in the French Quarter has led me down a research rabbit hole that’s rewriting everything I thought I knew about aging, longevity, and why certain myths refuse to die. Because sometimes the most outlandish legends are just science we haven’t figured out yet.
Recent scientific breakthroughs in heterochronic parabiosis, blood-borne rejuvenating factors, and aging mechanisms provide compelling theoretical frameworks that could explain how the consumption of young blood might have enabled Dracula to maintain his vitality across centuries.
Today’s blog is my attempt to examines the peer-reviewed scientific literature to construct a plausible biological rationale for the vampire’s legendary longevity, bridging ancient mythology with modern aging research to illuminate potential therapeutic targets for human healthspan extension.

Note: You will find a few phrases/words in every section hyperlinked in orangish color. These hyperlinks lead to the references, in case you want to verify and do some research on your own!
The Historical Context: From Mythology to Modern Science
The concept of blood as a life-preserving substance predates the original legend of Count Dracula by millennia. Medieval alchemists who pursued the search for elixir vitae (immortality serum) believed that human blood contained the essence of youth and vitality.

Medieval alchemical manuscript illustrating mystical processes related to blood and elixirs linked to life extension and immortality myths
While Bram Stoker (creator of Dracula, the 1897 Gothic horror novel) drew inspiration from the 15th-century Wallachian prince Vlad the Impaler, evidence suggests his vampire mythology was more heavily influenced by Irish folklore, particularly the legend of Abhartach, a Celtic chieftain who rose from the dead demanding “bowls of blood in recompense”. This confluence of historical bloodlust and supernatural regeneration established the foundational premise that blood consumption was believed to confer immortality.
The Parabiosis Paradigm: Scientific Validation of Blood-Mediated Rejuvenation
The scientific basis for blood-mediated rejuvenation emerged from heterochronic parabiosis experiments, where the circulatory systems of young and old animals are surgically connected. These groundbreaking studies revealed that aged mice sharing circulation with young counterparts experienced remarkable rejuvenation across multiple organ systems. The older animals demonstrated increased lifespan of 6-9%, equivalent to several additional years in human terms, along with improved cardiovascular function, enhanced muscle regeneration, and restored cognitive abilities.
Critically, this rejuvenation occurred through circulating blood factors rather than organ sharing alone. When researchers conducted controlled blood exchange experiments without anatomical connection, similar rejuvenating effects were observed, confirming that specific molecules in young blood possess anti-aging properties. These findings provide the scientific foundation that could theoretically explain Dracula’s centuries-long vitality through regular consumption of youthful blood.
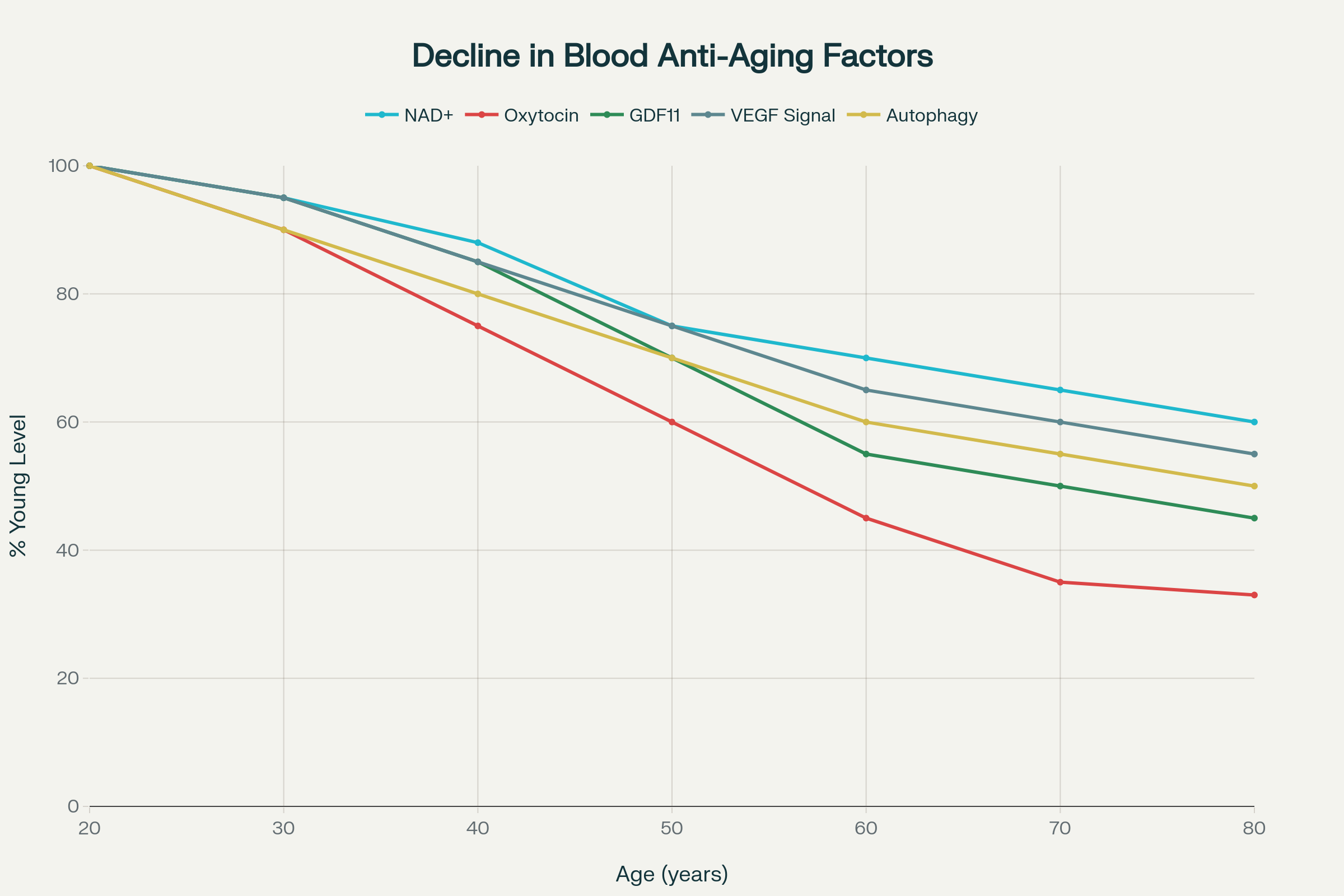
Age-related decline in blood-borne rejuvenating factors that could theoretically explain the benefits of young blood consumption in the Dracula myth
The Molecular Arsenal: Young Blood’s Anti-Aging Factors
Growth Differentiation Factor 11 (GDF11): The Systemic Rejuvenator
Among the most potent anti-aging factors identified in young blood is Growth Differentiation Factor 11 (GDF11), a member of the transforming growth factor-β superfamily whose levels decline significantly with age. Systemic administration of recombinant GDF11 to aged mice produced remarkable rejuvenation effects, including reversal of age-related cardiac hypertrophy, restoration of skeletal muscle stem cell function, and enhancement of neurogenesis.
Most remarkably, GDF11 treatment improved memory function and alleviated depression-like behaviors in aged mice through direct effects on hippocampal neurons, enhancing autophagy and reducing cellular senescence markers including p16INK4a and p19ARF by up to 70%. These effects occurred through mTOR pathway modulation, suggesting GDF11 acts as a master regulator of cellular aging processes.
For our Dracula and those mythological vampires, regular infusion of GDF11 through young blood consumption could theoretically maintain youthful cardiovascular function, muscle strength, and cognitive acuity across centuries.
Oxytocin: The Muscle Regeneration Hormone
Oxytocin, traditionally known for its role in social bonding and reproduction, emerges as a critical age-specific circulating factor essential for muscle tissue regeneration. Plasma oxytocin levels decline dramatically with age—by approximately 3-fold in elderly individuals—while oxytocin receptor expression in muscle stem cells similarly decreases. This decline contributes directly to age-related sarcopenia and impaired tissue repair capacity.
Systemic oxytocin administration to aged mice rapidly restored muscle regeneration by enhancing satellite cell activation through MAPK/ERK signaling pathways. Genetic knockout studies demonstrated that oxytocin deficiency causes premature sarcopenia, confirming its essential role in maintaining muscle tissue integrity.
For our Count Dracula, the abundant oxytocin present in young blood could maintain his supernatural strength and rapid healing abilities by preserving muscle stem cell function and tissue repair capacity.
VEGF and Angiogenic Factors: Vascular Fountain of Youth
Vascular Endothelial Growth Factor (VEGF) signaling represents another crucial component of young blood’s rejuvenating arsenal. Age-related VEGF signaling insufficiency underlies vascular aging and contributes to multi-organ dysfunction. Recent research demonstrates that modest increases in circulating VEGF were sufficient to preserve young-like vascular homeostasis and extend both lifespan and healthspan in aged mice.
Most intriguingly, aged human skin grafted onto young mice showed remarkable rejuvenation, with VEGF-A identified as both necessary and sufficient for this regenerative process. The rejuvenated skin exhibited improved aging biomarkers including reduced β-galactosidase, decreased p16ink4a expression, and increased SIRT1 and collagen 17A levels. This suggests that VEGF-rich young blood could theoretically maintain Dracula’s youthful appearance and tissue integrity through sustained angiogenesis and cellular repair mechanisms.
Extracellular Vesicles: Microscopic Messengers of Youth
Recent breakthrough research has identified small extracellular vesicles (sEVs) as key mediators of young blood’s rejuvenating effects. These nanosized messengers carry specific microRNA cargoes that can reprogram aged cells to a more youthful state. Intravenous injection of young sEVs into aged mice extended lifespan by 12.42%—equivalent to approximately 8 additional years in human terms.
The rejuvenating effects of young sEVs operate through delivery of specific microRNAs (miR-144-3p, miR-149-5p, and miR-455-3p) that stimulate PGC-1α expression, thereby enhancing mitochondrial function and cellular energy metabolism. This mechanism could explain how Dracula’s consumption of young blood provides not merely nutritional sustenance but actual cellular reprogramming toward a more youthful phenotype.
NAD+ and Metabolic Rejuvenation
Nicotinamide adenine dinucleotide (NAD+) represents a fundamental metabolic currency whose levels decline significantly with aging, particularly in males after age 40. NAD+ serves as an essential cofactor for sirtuins, a family of enzymes critical for longevity regulation, DNA repair, and stress resistance. The age-related decline in NAD+ contributes to mitochondrial dysfunction, reduced cellular energy production, and accelerated aging processes.
Young blood contains higher levels of NAD+ precursors and NAD+ biosynthetic enzymes that could theoretically restore cellular energy metabolism in aged tissues. The presence of extracellular NAMPT (eNAMPT) in young extracellular vesicles provides a mechanism for delivering NAD+ biosynthetic capacity directly to target cells. This could explain how Dracula’s blood consumption maintains his supernatural energy and vitality by continuously replenishing cellular NAD+ pools.
Cellular Senescence Reversal: Turning Back the Clock
One of the most significant discoveries in aging research is that old blood can induce cellular senescence in young organisms through circulating senescence-associated secretory phenotype (SASP) factors. Conversely, young blood contains factors that can reduce senescent cell burden and restore tissue function. The removal of senescent cells through senolytic interventions has been shown to extend healthspan and reduce age-related pathologies.
Young blood’s anti-senescence effects operate through multiple mechanisms including reduced inflammatory cytokines (IL-6, TNF-α, IL-1β), decreased oxidative stress, and enhanced autophagy. These effects could theoretically explain how Dracula’s regular consumption of young blood prevents the accumulation of senescent cells that would otherwise drive his aging and eventual demise.
Autophagy Enhancement: Cellular Quality Control
Autophagy, the cellular process responsible for removing damaged proteins and organelles, declines significantly with age. This decline contributes to the accumulation of cellular damage and age-related dysfunction. Young blood contains factors that can enhance autophagy through mTOR pathway modulation and TFEB activation.
The GDF11 present in young blood specifically stimulates autophagy in aged neurons, leading to improved cellular function and reduced senescence markers. Additionally, young blood contains spermidine and other polyamines that can restore autophagy capacity in aged immune cells. For Dracula, these autophagy-enhancing factors could maintain cellular quality control systems, preventing the accumulation of damaged proteins and organelles that typically drive aging processes.
The Blood-Brain Barrier: Maintaining Cognitive Vitality
Age-related decline in blood-brain barrier (BBB) function contributes to cognitive decline and neurodegeneration. While some studies suggest BBB permeability increases with aging, the relationship is complex and region-specific. Young blood factors, particularly VEGF and other angiogenic molecules, can help maintain BBB integrity and support cerebral vascular health.
The presence of young extracellular vesicles that can cross the BBB and deliver rejuvenating microRNAs directly to brain cells provides a mechanism for cognitive preservation. This could explain how Dracula maintains his intellectual acuity and strategic thinking abilities across centuries, as his regular consumption of young blood would continuously supply BBB-protective factors and neural rejuvenating signals.
Inflammatory Regulation: Suppressing Inflammaging
Chronic low-grade inflammation (inflammaging) represents a hallmark of aging that contributes to multiple age-related pathologies. Young blood contains anti-inflammatory factors and exhibits lower levels of pro-aging cytokines compared to aged blood. The inflammatory aging clock (iAge) has emerged as a powerful biomarker that correlates with multimorbidity and cardiovascular aging, with the chemokine CXCL9 identified as a key contributor to endothelial senescence.
Young blood’s anti-inflammatory effects could theoretically suppress inflammaging in Dracula’s tissues, preventing the cascade of inflammatory damage that typically accelerates aging. The presence of young immune cells and anti-inflammatory extracellular vesicles would continuously counteract age-related inflammatory signaling, maintaining tissue homeostasis and function.
Sirtuins: The Longevity Enzymes
Sirtuins, particularly SIRT1, represent evolutionarily conserved regulators of aging and longevity. These NAD+-dependent enzymes promote DNA repair, enhance stress resistance, and regulate metabolic processes essential for healthy aging. Young blood contains higher levels of NAD+ and sirtuin-activating factors that could theoretically maintain sirtuin activity in aged tissues.
Brain-specific SIRT1 overexpression has been shown to significantly extend lifespan in mammals, suggesting that sirtuin activation in neural tissues is particularly important for longevity. The NAD+ precursors and sirtuin activators present in young blood could maintain these longevity pathways in Dracula’s tissues, providing a molecular basis for his extended lifespan.
Telomere Maintenance: Preserving Chromosomal Integrity
Telomere shortening represents a fundamental mechanism of cellular aging, with critically short telomeres triggering senescence and contributing to age-related pathologies. While telomere length in blood serves as a biomarker of biological age, the relationship between telomeres and aging is complex and varies across different cell types.
Some evidence suggests that centenarians maintain longer telomeres and higher telomerase activity compared to younger individuals, possibly representing a survivor effect. Young blood may contain factors that support telomere maintenance or enhance telomerase activity, though this remains an area of active investigation. For Dracula, theoretical telomere preservation through young blood factors could contribute to his cellular immortality by preventing the chromosomal deterioration that typically limits cellular lifespan.
Systemic Integration: The Holistic Rejuvenation Effect Of Young Blood
The remarkable aspect of young blood’s rejuvenating effects lies in their systemic and coordinated nature across multiple organ systems. Rather than targeting isolated pathways, young blood appears to restore youthful function through integrated networks involving metabolism, inflammation, vascular health, cellular repair, and neural function.
This systemic rejuvenation could explain how Dracula maintains not just extended lifespan but preserved vitality and function across centuries. The coordinated effects of GDF11, oxytocin, VEGF, NAD+ precursors, anti-inflammatory factors, and extracellular vesicles would theoretically maintain his cardiovascular health, muscle strength, cognitive abilities, and physical appearance in a youthful state.
Clinical Implications and Therapeutic Potential
The scientific principles underlying Dracula’s theoretical blood-mediated longevity have profound implications for human aging intervention. Current clinical trials are investigating young plasma infusions for age-related diseases. The complexity of young blood’s rejuvenating effects suggests that isolated factor supplementation may be insufficient, requiring more comprehensive approaches.
Extracellular vesicle therapies represent a particularly promising avenue, as they can deliver multiple rejuvenating factors simultaneously while avoiding the complications associated with whole blood transfusions. The identification of specific microRNAs and proteins responsible for anti-aging effects enables the development of targeted interventions that could capture young blood’s benefits without its risks.
Limitations and Considerations
While the scientific evidence provides compelling theoretical support for blood-mediated rejuvenation, several limitations must be acknowledged. The majority of research has been conducted in rodent models, and translation to human aging may involve additional complexities. The optimal dosing, frequency, and duration of young blood factor interventions remain unclear.
Furthermore, the heterogeneity of aging processes across individuals suggests that personalized approaches may be necessary. The potential for immune reactions, pathogen transmission, and unintended consequences must be carefully considered in any therapeutic applications. For Dracula, his supernatural physiology may circumvent these limitations, but human applications would require extensive safety validation.

Recently, several companies have begun marketing “young blood” infusions or transfusions as anti-aging treatments, claiming that plasma from younger donors can rejuvenate tissues and slow age-related decline. These offerings have sparked considerable ethical concerns, with critics raising issues about potential exploitation of young donors, unproven claims, and possible health risks for recipients. The practice has drawn regulatory scrutiny; the FDA has issued warnings and crackdowns against clinics promoting young plasma for aging without robust scientific evidence, emphasizing that such treatments are not approved and may pose serious safety risks. This regulatory action highlights the need for rigorous clinical validation and ethical oversight before new interventions targeting human aging are embraced.
Future Research Directions
The convergence of vampire mythology and aging science opens several avenues for future investigation. Single-cell RNA sequencing of tissues exposed to young blood factors could elucidate cell-type-specific rejuvenation mechanisms. Longitudinal studies tracking biomarkers of aging in response to young blood factor interventions would provide crucial translational insights.
The development of young blood factor mimetics or synthetic alternatives could overcome the practical limitations of blood-derived therapies. Additionally, investigating the molecular mechanisms underlying successful aging in centenarians may reveal endogenous pathways that parallel young blood’s effects, providing targets for pharmaceutical intervention.
Conclusion
The scientific examination of Dracula’s blood consumption reveals a remarkably sophisticated biological strategy for longevity maintenance. The convergence of GDF11-mediated tissue regeneration, oxytocin-driven muscle preservation, VEGF-sustained vascular health, NAD+-supported cellular metabolism, autophagy enhancement, senescence suppression, and extracellular vesicle-mediated cellular reprogramming provides a comprehensive theoretical framework for achieving extended healthspan and lifespan.
While Bram Stoker could not have anticipated the molecular mechanisms now being elucidated by modern biogerontology research, his intuitive understanding of blood as a life-sustaining force proves remarkably prescient. The myth of Dracula serves not merely as gothic legend but as an inadvertent blueprint for the most promising therapeutic strategies emerging from contemporary aging research.
The vampire’s ancient secret—that young blood contains the essence of vitality—now stands validated by peer-reviewed science. As researchers continue to unravel the molecular mechanisms underlying young blood’s rejuvenating effects, the boundary between supernatural fiction and scientific possibility continues to blur, offering hope that humanity may one day harness these same pathways to extend not just lifespan, but the quality and vitality of our extended years.
The enduring appeal of the Dracula myth reflects humanity’s fundamental desire to transcend the limitations of aging and mortality. Through rigorous scientific investigation of young blood’s anti-aging properties, we move closer to transforming this ancient dream into therapeutic reality, albeit through medicine rather than mythology.
If you have read this article, I have a question for you to ponder on:
If tomorrow you happen to receive blood transfusion, will you worry if that blood came from an older donor? Think!





Subir is a brilliant story teller. He connects science with beliefs and philosophy in a marvelous manner. In this article we need to understand that these are only correlations: drinking blood had not been real: all factors/ proteins get degraded inside the intestine before they are absorbed in the blood stream.
According to this reasoning, cord blood may be preferable; however, its quantity and availability are limited.